After Chengshuo Hardware mechanical engineers have completed precision machining and prototype size testing of metal products, our product processing department will perform more refined post-processing of metal products according to the environment in which customers use metal products.
Many people think of surface treatment, and they may only consider it as an aesthetic finish like paint and powder coating to make the parts look more beautiful and change color. In fact, surface treatment is not just for aesthetics. Various surface treatments treat the exterior of metal products by applying a thin supplementary layer on the surface. Appropriate surface treatment can help different types of metal precision processed products receive better protection in the usage environment (such as corrosion resistance, slowing down rust), protect metal products, and achieve the goal of extending the service life.
Today we will introduce to you the aluminum product production and surface treatment, anodizing, which Chengshuo Hardware is particularly skilled at.
What is anodizing?
Anodizing is an electrochemical process that converts a metal surface into a decorative, durable, and corrosion-resistant anode oxide surface. Aluminum is very suitable for anodizing, although other non-ferrous metals such as magnesium and titanium can also be anodized.
In 1923, anodizing was first applied on an industrial scale to protect aluminum components of seaplanes from corrosion. In the early days, chromic acid anodizing (CAA) was the preferred process, sometimes referred to as the Bengough Stuart process, as described in the UK Defence Specification DEF STAN 03-24/3.
The current popular classification of anodizing
Anodizing has been widely used in industry for a long time. There are many ways to use different names, and there are several classification methods that can be summarized as follows:
Classified by current type: DC anodizing; AC anodizing; And pulse current anodizing, which can shorten the production time to achieve the required thickness, make the film layer thick, uniform and dense, and significantly improve corrosion resistance.
According to the electrolyte, it can be divided into sulfuric acid, oxalic acid, chromic acid, mixed acid, and naturally colored anodic oxidation with sulfonic organic acids as the main solution. Oxalic acid anodizing was patented in Japan in 1923 and later widely used in Germany, especially in construction applications. Anodized aluminum oxide extrusion was a popular building material in the 1960s and 1970s, but was later replaced by cheaper plastics and powder coatings. Various phosphoric acid processes are one of the latest developments in the pre-treatment of aluminum parts used for bonding or painting. The various complex changes in the anodic oxidation process using phosphoric acid are still evolving. The trend of military and industrial standards is to classify anodizing processes based on coating characteristics in addition to identifying process chemistry.
According to the properties of the film layer, it can be divided into: ordinary film, hard film (thick film), ceramic film, bright modification layer, semiconductor barrier layer, etc. for anodizing.
Classification of Anodizing Processes for Aluminum Products
Anodizing process is sometimes used for exposed (non coated) aluminum machined or chemically milled parts that require anti-corrosion protection. Anodic coatings include chromic acid (CAA), sulfuric acid (SAA), phosphoric acid, and boric acid sulfuric acid (BSAA) anodizing processes. The anodizing process involves the electrolytic treatment of metals, in which a stable film or coating is formed on the metal surface. Anodic coatings can be formed on aluminum alloys in various electrolytes using either alternating current or direct current.
Anodizing is achieved by immersing aluminum in an acidic electrolyte bath and passing current through the medium. The cathode is installed inside the anodizing tank; Aluminum acts as an anode, releasing oxygen ions from the electrolyte and binding to aluminum atoms on the surface of the anodized portion. Therefore, anodizing is a highly controllable oxidation that enhances natural phenomena.
Anodization includes Type I, Type II, and Type III. Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of aluminum parts. Aluminum components are anodized (hence referred to as “anodizing”), and current flows between them and the cathode (usually a flat aluminum rod) through the aforementioned electrolyte (most commonly sulfuric acid). The main function of anodizing is to increase corrosion resistance, wear resistance, adhesion to paint and primer, etc
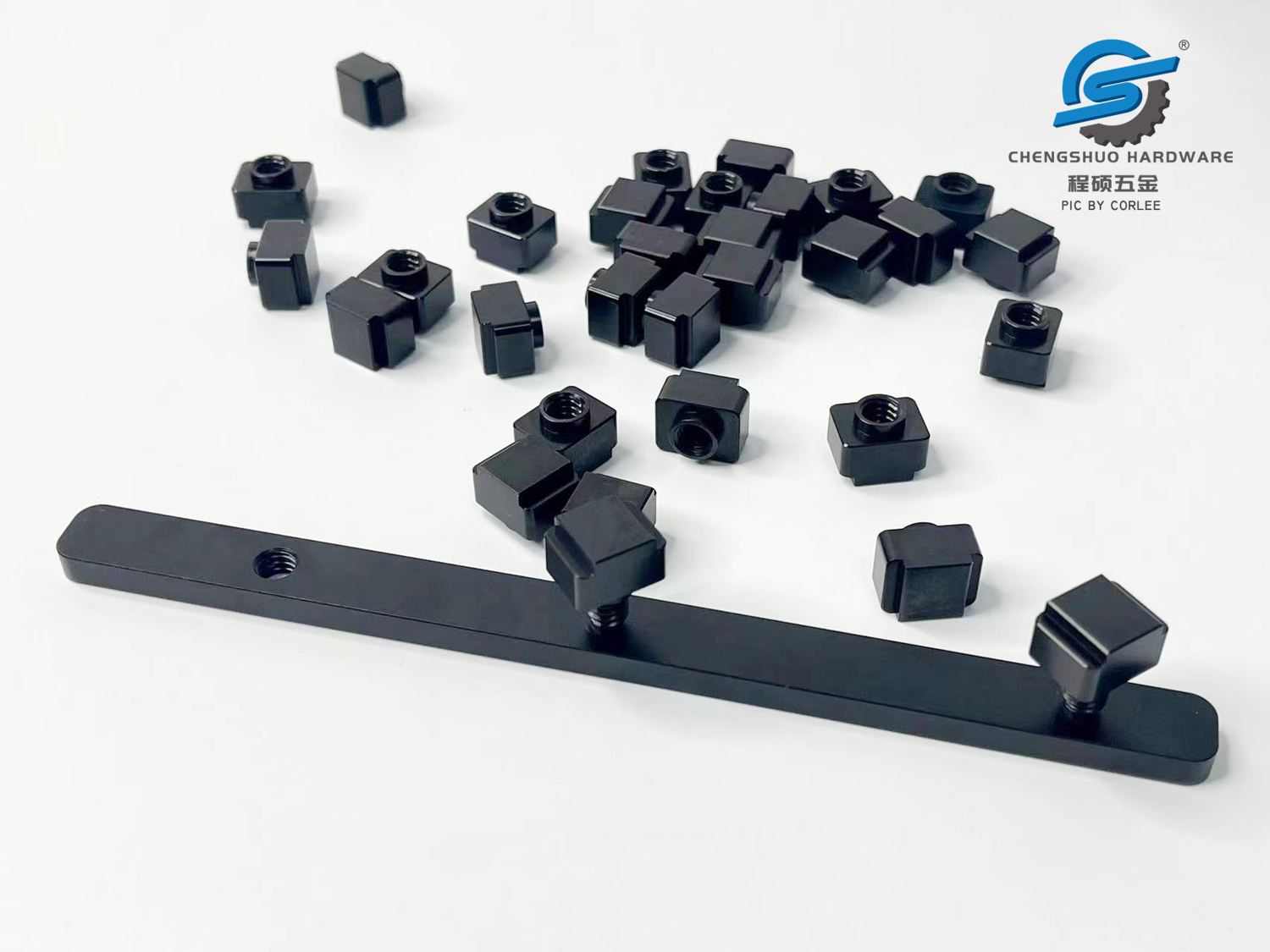 PIC by Corlee: Type III anodized aluminum parts
PIC by Corlee: Type III anodized aluminum parts
The anode oxide structure originates from an aluminum substrate and is entirely composed of aluminum oxide. This type of alumina is not applied to the surface like paint or coatings, but is completely integrated with the underlying aluminum substrate, so it will not shatter or peel off. It has a highly ordered porous structure and can be subjected to secondary processing such as coloring and sealing.
Post time: May-29-2024


